House Keeping
This is great news for permitting times, as the EPA also announced they now have 73 projects waiting to be federally permitted.
Recap
In this storage soirée, we’ve covered the creation of the underground injection control (UIC) program at the EPA, most notably within that Class VI wells for dedicated CO2 storage. We also took a peek at the latest legislation coming out of the US and the resulting investment in our subsurface. We have set the playing field for a future full of high-quality, safe CO2injection. And yet I want more.
There is always better policy, more guardrails, and sexier geologies to consider for any set of problems, storage of CO2is no different. I want to explore what one of those geologies looks like. What will the Class VI program look like in 2030?
New(ish) Geology
As we discussed, the Class VI well system was created with a specific geology in mind: sedimentary basins. Sedimentary basins or reservoirs are region-scale depressions of the Earth's crust where subsidence has occurred, and a thick sequence of sediments has accumulated to form a large three-dimensional body of sedimentary rock. Drilling down even further, we find that we are targeting sedimentary basins and aquifers: A water-bearing stratum of permeable rock, sand, or gravel. Important here due to sedimentary basins having brines (water and dissolved solids) between grains.
Once injected, the CO2 plume stays underground, being trapped by the seal unit (structural or stratigraphic trapping), between the grains (residual), dissolved in the brine (solubility) or a phase change from supercritical into a solid carbonate (mineral), this process is also referred to as “mineralization.”
I am a sandstone lover as much as the next storage enthusiast, and they have borne the literal and figurative weight of nearly all CO2 storage to date, more than 300+ million tons of CO2 stored safely! However, we need to look past the humble sandstone and sedimentary basins to imagine a world with more geologies to inject and more capacity to store. We also need some regulatory changes to accommodate these new geologies. We need to look toward igneous formations.
Igneous Rocks
Sedimentary basins are wonderful geologies for CO2 injection, but we shouldn’t limit our reservoirs. We should look to other geologies with unique prosperities that make them attractive for injection, enter the igneous.
Igneous or magmatic rock is one of the three main rock types, the others being sedimentary and metamorphic. Igneous rocks are formed through the cooling and solidification of magma or lava.
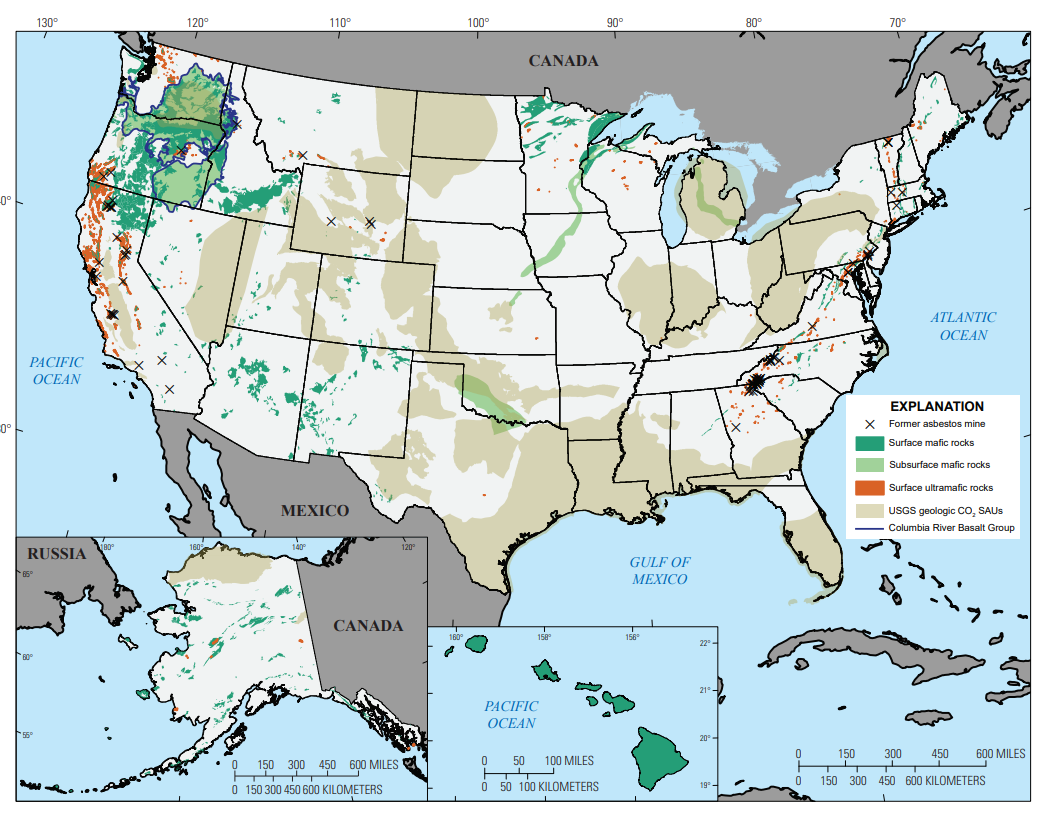
A particular set of igneous rocks known as mafic or ultra-mafic are particularly attractive. A mafic mineral or rock is a silicate mineral or igneous rock rich in magnesium and iron. The rocks most frequently used for CO2 injection are basalts and peridotites, but other mafic/ultra-mafic rocks include olivine, diabase, gabbro, dunites, serpentinites.
The Mg, Ca, and Fe ions of mafic/ultra-mafic are important because they react with CO2 to form a solid carbonate. These reactions are faster than sandstone/sedimentary basins. A reminder this kind of reaction results in mineralization or mineral trapping.
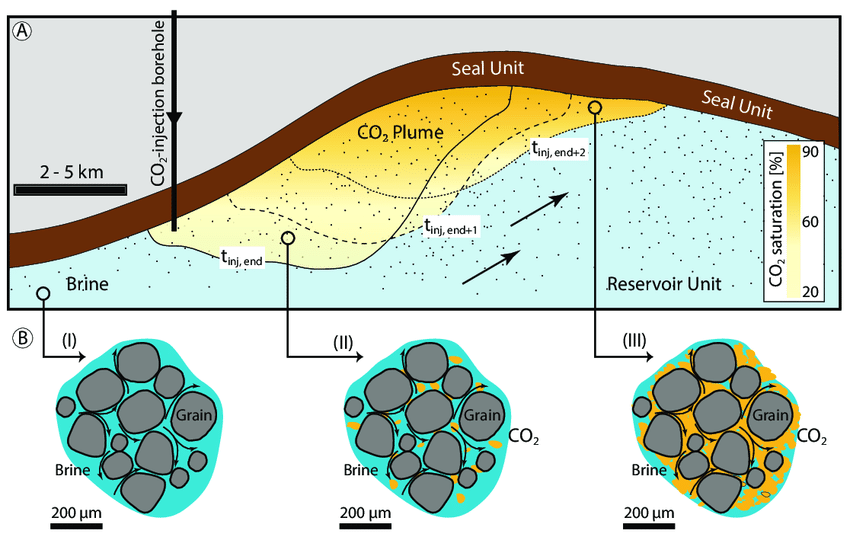
The below figure demonstrates the extent of each trapping mechanism is highly site-specific and depends on several parameters, including the type of rock: carbonatitic and siliciclastic rocks (left panel) or mafic and ultramafic rocks that can react much faster with CO2 to form carbonates (right panel)
Reaction rates and carbonate formation matter, because once a carbonate has formed the CO2 stays put. This reduces the need for expensive long-term monitoring regimes and ensures the permanence of the CO2 since sublimation is not a likely outcome.
But even within mafic / ultra-mafic rocks, not all reaction rates are the same!
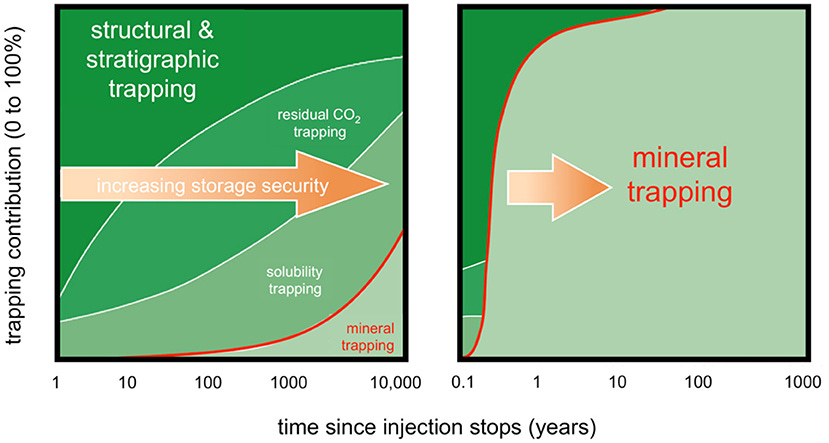
This figure shows the varying reaction rates, and thus carbonate formation, for CO2 injected underground (in-situ) vs. above ground (ex-situ or surficial). We can see that peridotite in a CO2-rich fluid circulation react more quickly than basalts in the same fluid and much more quickly than sedimentary rocks with supercritical CO2.
From National Academies of Sciences Engineering Medicine (2019) Mine tailings are gray, peridotite in dark green, basalt in purple, brucite in light green, and sedimentary rocks in orange.
Not only do mafic and ultra-mafic react faster, but they are abundant, and in some cases, cost-competitive with sedimentary basins.
This figure summarizes the cost of CO2 stored (US$/tCO2) vs. the storage potential of CO2 per year (GtCO2/yr). Red boxes illustrate costs and rates for ex-situ CO2 mineralization using heat and concentrated CO2. Yellow boxes are for surficial CO2 mineralization of mine tailings, ground peridotite added to soils or beaches, and peridotite mined and ground for CO2 removal from the air with solid storage. Green arrows are for in-situ carbon storage by injecting CO2-enriched fluids into mafic and ultramafic formations. Blue arrows are for in-situ carbon sequestration by circulating water saturated in the air into peridotite formations for CO2 removal from the air with solid storage. The gray arrow is for in-situ carbon sequestration by injecting supercritical CO2 into subsurface sedimentary formations. Figure modified from (National Academies of Sciences Engineering Medicine, 2019),
We have the academic data, and we have companies doing it right now. Carbfix is injecting CO2 dissolved in water into the basalts of Iceland, 44.01 is injecting into peridotites in Oman and the UAE next, and a new company Cella is injecting CO2 into volcanic rock in Kenya!
You may notice something about all those locations; none are in the US. And that’s a problem.
Before we get to the fix, I want to acknowledge that everything in this post is from researchers who have dedicated their lives to this. I am not a geologist, but a rock enthusiast inspired by the work of Dr. Greg Dipple, Dr. Peter Kelemen, Dr. Sally Benson, Dr. Peter Psarras, Dr. Helene Pilorge, Dr. Siobhan Wilson, Dr. Natalia Zakharova, Dr. Elisabet Ragnheidardottir, Dr. Jen Wilcox, and many more, who have laid the groundwork for mineralization to become a viable storage technology today. Without them, none of this is possible.
A New Class
The Class VI regulatory regime was written for sedimentary basins because that is the rock we have been injecting into for most of history. We have the data, the historical success and the safety record to justify it. No actual language says a Class VI well has to be a sedimentary basin. However, some critical distinctions of the Class VI program need to be modified, without sacrificing any safety, to best capitalize on the benefits of igneous geologies.
Injection of CO2 as a seltzer / dissolved in water / sub-critical
To date, all CO2 injected into Class VI wells has been supercritical. Many of the companies injecting into mafic/ultra-mafic formations use CO2 dissolved in water to speed up reaction times. The EPA has made no definitive statement on whether or not they would allow CO2 dissolved in water (also called a seltzer or broadly sub-critical) to be injected into a Class VI well. They have said they evaluate each application “on its own merits,” but a clear distinction would help eliminate the uncertainty.
Caprock requirement
Current Class VI regulations require a caprock or sealing unit. This unit will physically trap the CO2 as it matriculates through the storage unit due to buoyant forces, a crucial safety feature. However, those same buoyant forces are less relevant when CO2 is dissolved in water since it is denser than the surrounding fluids.
Number injection wells
Due to several factors (porosity, permeability, injection rates), injection into mafic/ultra-mafic may take more injection wells than sedimentary basins. It is unclear if the EPA would allow several injection wells, and they again have said all projects are evaluated on their own merits.
There are other issues around what type of monitoring, reporting, and verification methods are necessary, especially since the mineralization happens much faster than in sedimentary basins, and Carbfix has done extensive work in that area.
Options to give in-situ mineralization a dedicated pathway:
Changes to current Class VI regulations to account for differences between sedimentary and igneous geologies
A Class VI(b) well for in-situ mineralization
A new class VIII well distinction, Class VII potentially for geothermal
Regardless of the mechanism, the geologies, injection methods, and MRV are different enough from sedimentary basins to warrant a dedicated regulatory pathway.
This pathway increases US storage capacity by gigatons. The US has rich basalt and peridotite deposits, which can be used for storage.
Before any of the regulatory changes are underway, we need US-based geologic data, to validate our specific geologies are suitable for injection. Above all else we must ensure that drinking water will be protected and that there is little to no risk of reversal.
And lucky for us, the Department of Energy (DOE) is doing just that by giving a $10.5 million grant to researchers in the UW School of Energy Resources (SER) Center for Economic Geology Research (CEGR) will lead the HERO Basalt CarbonSAFE (Hermiston Oregon Carbon Storage Assurance Facility Enterprise) project in partnership with Oxy Low Carbon Ventures (Oxy), Pacific Northwest National Laboratory (PNNL) and Calpine to accelerate the scale-up and deployment of commercial CO2 storage in basaltic rocks at a storage complex near Hermiston, Ore.
This is on top of a previous, DOE funded project, in the basalts of Walla Walla, WA.
A Storage State of Mind
After espousing the virtues of mafic/ultra-mafic geologies, I need to note that these geologies are not necessarily better or worse than sedimentary basins; they’re different. In some cases, the mineralization of mafic/ultra-mafic happens so quickly it can cause issues. You can get mineralization into your borehole, blocking new injections of CO2-rich fluid or portions of your reservoir.
There are challenges with in-situ mineralization that need to be studied and understood. Still, provided they can meet the safety requirements set forth by the Class VI program, we should take advantage of the geophysical attributes of these geologies in our race against climate change. They provided a valuable and essential tool in our climate change kit.
A dedicated regulatory pathway does not ensure that in-situ mineralization would scale, but it does ensure that it has the opportunity to scale. Sedimentary basins will continue to enjoy the lion's share of CO2 injection in the short to medium-term. We can move in concert to ensure all geologies that demonstrate the safe storage of CO2 is available for injection. Why limit our storage capacity?
This ends my Class VI series; I hope you enjoyed it. Next up is carbon dioxide removal and the policy support it needs to meet the gigaton per year challenge.


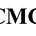


excellent post... thanks so much and appreciate the reference to the paper: An Overview...
Hi Jack,
Great post as always.
I imagine storage and minerlisation requires a high level of permeability, this is obvious in sedimentary rocks but how does this occur within crystalline mafic rocks? Are they highly fractured to increase contact area?